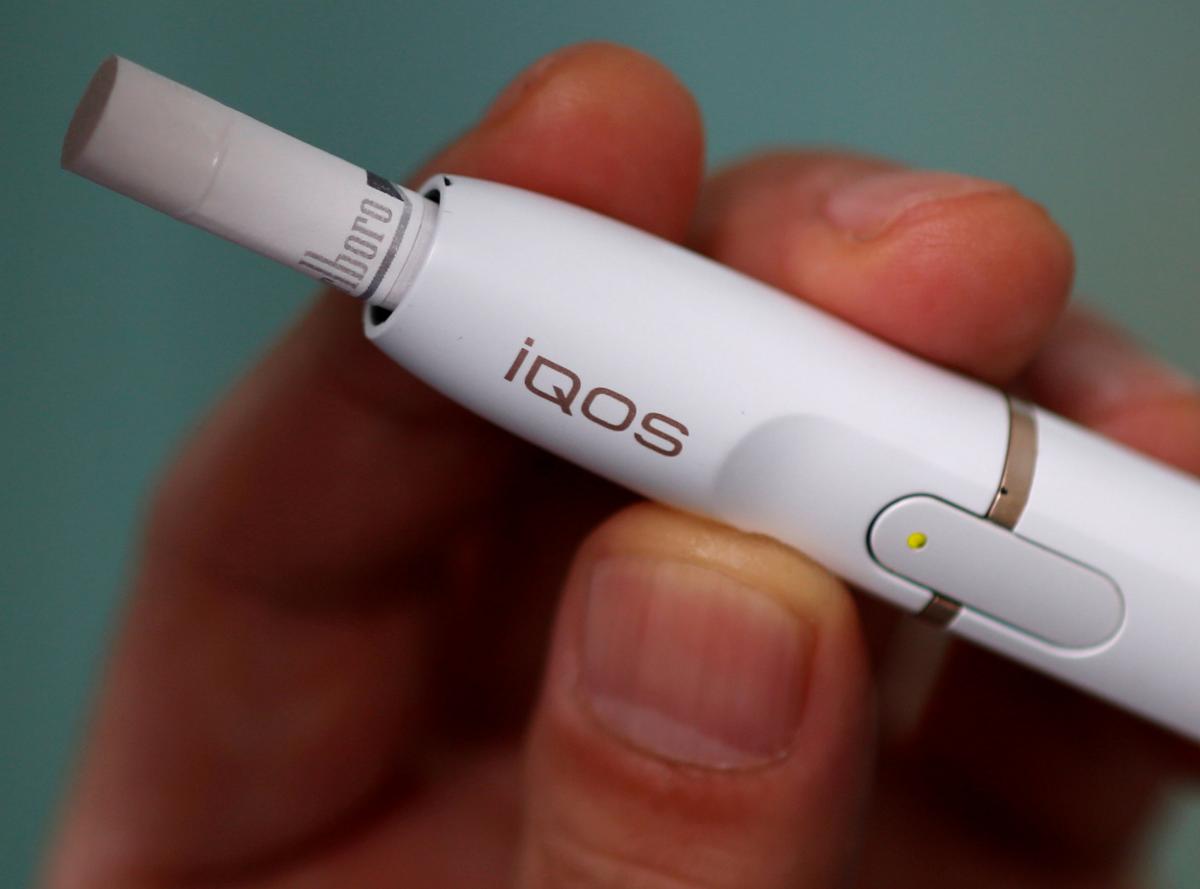
WASHINGTON (Reuters) – In a decision expected to test the Trump administration’s approach to tobacco regulation, U.S. health advisers will vote this week on whether to allow Philip Morris International Inc (PM.N) to sell its novel iQOS tobacco device and claim it is less harmful than cigarettes.
The sleekly packaged little tube would not look out of place in an Apple store. It is designed to heat tobacco but not burn it. Most of the harmful chemicals in tobacco are released when tobacco is burned.
The advisers to the Food and Drug Administration will discuss the product on Wednesday and Thursday and recommend whether it should be cleared. The FDA is not obliged to follow the recommendations of its outside advisers but typically does.
If cleared, iQOS would become the first product to carry a modified-risk claim and could help advance the FDA’s proposed new approach to reducing the dangers of smoking.
For decades, U.S. health agencies have worked to help Americans quit cigarettes to avoid the risks of lung cancer and other disease. National smoking rates have declined to near historic lows of around 15 percent.
But in July, newly appointed FDA Commissioner Scott Gottlieb proposed reducing nicotine levels in cigarettes to “non-addictive” levels while increasing development of lower-risk alternatives. The policy assumes that some percentage of the population will be unable or unwilling to give up nicotine.
To make the new strategy succeed, the agency needs a stable of vetted, reduced-risk alternatives to cigarettes on the market. Philip Morris is one of the few companies that can finance such a long and expensive development process. It has spent close to $3 billion on reduced-risk products.
To sell a new tobacco product, a company must demonstrate that it significantly reduces the risk of disease and does not encourage more smoking or delay quitting.
To date, the FDA has determined that only eight products meet that standard, all of them snus smokeless tobacco pouches made by Swedish Match AB (SWMA.ST). The agency has granted no company the right to specifically claim that their product is less risky than cigarettes.
Tobacco control activists say companies like Philip Morris cannot be trusted to make reduced-risk claims. They point to the industry’s previous promotion of “light” and “low-tar” cigarettes as safer alternatives despite evidence showing they were not.
Others argue that things have changed. Since 2009, the tobacco industry has had to answer to the FDA under federal law. New technology, including e-cigarettes and heat-not-burn products, may help smokers quit or shift those who cannot quit onto a less harmful substitute, they say.
“Many of my friends in the tobacco control movement are still fighting the tobacco wars of the 1980s,” said Scott Ballin, an independent health policy consultant and long-time anti-tobacco activist. “We are beyond that now.”
The FDA’s Gottlieb favors an approach to treating addiction that makes available less harmful versions of addictive substances, including nicotine and opioids. These products, he argues, can help minimize damage to those unable to quit.
Mitch Zeller, head of the FDA’s tobacco division, refers frequently to a “continuum of risk” for nicotine products, with nicotine gums and lozenges on one end and cigarettes on the other. The FDA would like to shift smokers unable to quit nicotine from high-risk to low-risk products.
“What we have developed is an offering on the continuum of risk,” said Ruth Dempsey, Philip Morris’ director of scientific engagement. “We believe it is better than cigarettes.”
 0 comments
0 comments





